Fast-Track Canadian Patents
The Canadian Intellectual Property Office has announced its participation in the Canada-U.S. Patent Prosecution Highway (PPH) pilot project. This experimental program, which has been in the works for some time, is designed to help fast-track patent examination in both Canada and the U.S. and increase efficiency in the patent examination process. The PPH project is also being used by other intellectual property offices, including the Japan Patent Office, the United Kingdom Intellectual Property Office, and the Korean Intellectual Property Office. During the initial one-year pilot period, CIPO won’t require a fee to process requests for accelerated examination under the PPH project.
Â
Calgary – 10:45 MST
No commentsiPhone Patent Infringement Suit
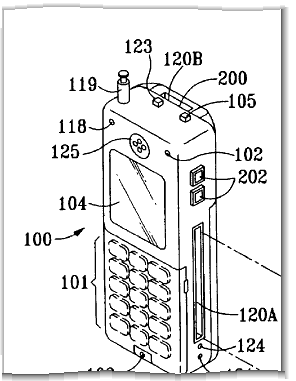 It was only a matter of time before someone took a run at Apple’s famous iPhone and it is reported that Minerva Industries, Inc. launched a patent infringement lawsuit against Apple on Tuesday, mere hours after the unknown company was told that its US patent issued (Patent No. 7,321,783 entitled “Mobile Entertainment and Communication Device”). A portion of figure 1 from the patent is excerpted at left.
It was only a matter of time before someone took a run at Apple’s famous iPhone and it is reported that Minerva Industries, Inc. launched a patent infringement lawsuit against Apple on Tuesday, mere hours after the unknown company was told that its US patent issued (Patent No. 7,321,783 entitled “Mobile Entertainment and Communication Device”). A portion of figure 1 from the patent is excerpted at left.
From reading the claims, this inventor should have every cell-phone maker on the planet running scared. Something tells me the cell-phone industry will try and compare notes on this one to dig up some prior art. Apple is no stranger to IP lawsuits over its iPhone. After all, the product was launched in the midst of a highly-publicized trade-mark infringement fight.
Calgary – 21:35 MST
No commentsUS Patent Reform
An interesting article in the NY Times gives an update on the US patent reform process and the battle lines that are drawn between large technology companies such as Intel, Microsoft, IBM and Apple, who are looking for ways to take away leverage from small-time patent holders, and the pharmaceutical industry which traditionally relies on robust patent protection.’
And if you want some more light reading, try the draft report prepared by the Senate Judiciary Committee on the Patent Reform Act of 2007.
Â
Calgary – 15:25 MST
1 commentIP and Internet Law in 2008
Four intellectual property topics to watch in the new year:
Canadian Copyright Changes
The government’s announcement of new copyright legislation has generated considerable debate in Canada in the last few weeks – when copyright reform hits the front page of the Globe and Mail, then you know something is up.  So far all of the media interest, including a Facebook group of several tens of thousands of concerned citizens, seems to have triggered a review of the proposed legislation. We will be watching to see what the bill looks like.
Filesharing
We are hoping to see some clarity in this area in 2008. The recording industry seems to be pushing for an opportunity to litigate, and the proposed copyright legislation may address this issue, providing guidance to content-providers, musicians, users and industry alike.
US Patent Reform
The proposed patent reform bill in the US is currently making its way through Capitol Hill and the changes effected by the new law will undoubtedly transform the patent landscape. We’ll keep an eye on these developments south of the border because of their impact on so many Canadian patent holders and inventors.
Privacy Online
Privacy issues will continue to play a central role in the conduct of online business. Massive privacy breaches have become almost common-place – announcements of lost data or hacked credit card info now seem to be weekly occurences - and Canadian law in this area is changing as new decisions and investigations try and produce guidelines for business. Watch for this area to continue to evolve over the next year.
Â
Calgary – 10:35 MST
No commentsOpen Source Resources
There seems to be a run on BusyBox litigation this fall and the lawyers at the Software Freedom Law Center (SFLC) have been busy; last week they filed another copyright infringement lawsuit against Verizon Communications, Inc., alleging violation of the GNU General Public License (GPL). The open source movement is expanding past the world of software into areas of biological and life sciences. Because of the growing interest in this area, we have decided to compile a list of resources regarding open source organizations and law.
Software
Software Freedom Law Center: http://www.softwarefreedom.org/
Free Software Foundation: http://www.fsf.org/
Open Source Initiative: http://www.opensource.org/Â
Biotech / Life Sciences
The BiOS Initiative:http://www.bios.net/
BiOS Compatible Agreement Listing:
http://www.bios.net/daisy/bios/licenses/2997.html
Public Intellectual Property Resource for Agriculture: http://www.pipra.org/
Tropical Disease Initiative: http://www.tropicaldisease.org/
WCLP: http://www.westcoastlicensing.com/index.htmÂ
AFMnet: http://www.afmnet.ca/Â
LES Presentation Slides: lespresentation-open-source.pdf
Calgary – 16:00 MST
Â
2 commentsInfringement of Patent on Higher Life Form
The decision earlier this year in Monsanto Canada Inc. v. Wouters 2007 FC 625 (Barnes, J.) marks the first damage award in a case pitting Monsanto against a farmer in Canada. In the 2004 landmark case of Monsanto Canada Inc. v. Schmeiser, 2004 SCC 34, [2004] 1 S.C.R. 902, Monsanto sued a Saskatchewan farmer for unauthorized use of Monsanto’s patented Roundup Ready Canola, a canola variety containing genetically modified genes and cells. The court decided that Schmeiser had infringed Monsanto’s patent by growing the plants on his farm, but Schmeiser “did not gain any agricultural advantage from the herbicide resistant nature of the canola since no finding was made that they sprayed with Roundup herbicide to reduce weeds.” In short, the farmer earned no profit from the patented invention and Monsanto was not entitled to any compensation for the infringement.
In the Wouters case, the Ontario farmer may have been able to raise the Schmeiser defence (or any number of other defences). Problem is, Wouters never filed a proper defence, leaving the court with little choice but to declare Monsanto the winner by default. The court concluded that Wouters infringed the patent by growing, harvesting and selling 392 acres of soybeans which Wouters knew contained the patented genes and cells. The resulting damage award of over $100,000 appears to mark the first award for infringement of a patent covering a so-called “higher life form” in Canada.
Yes, the Supreme Court of Canada in the famous Harvard Mouse Case decided clearly that “higher life forms” are not patentable in Canada. However, the same court in Schmeiser had to admit that the individual cells are patentable, and the patented genes and cells are not merely a part of the plant; rather, the patented cells compose its entire physical structure. So the court may not want to permit the patenting of higher life forms, but the higher life forms are inseparable from the patented cells of which they are comprised. You can’t destroy all the cells and leave the plant alive.
Life forms have a way of getting up and moving around: seeds propagate and blow away, creatures crawl, viruses replicate and invade. Where will the Monsanto cases go when they grow legs and start crawling around the patent landscape?Â
Â
Calgary – 09:35 MST
USPTO Releases Post-KSR Guidelines
We wrote about the US Supreme Court decision in KSR International Co. v. Teleflex Inc. in our earlier post. The USPTO has just released Examination Guidelines to aid examiners in determining obviousness in the wake of the KSR decision.
Calgary – 22:50 MST
No commentsPfizer Wins Canadian Patent Skirmish
U.S.-based drug-maker Pfizer scored a win this month at the Canadian Federal Court in its fight with Ranbaxy. Ranbaxy, a generic drug manufacturer based in New Delhi, is India’s largest pharma company.  The Canadian court granted Pfizer’s application for an order preventing Ranbaxy from launching its generic product in Canada until expiration of Pfizer’s patent in 2016. It is expected that Ranbaxy will appeal this decision. Ranbaxy has enjoyed some success in other jurisdictions in getting its generic products approved.
In Canada and the U.S., billions of dollars worth of patented drugs will expire or go “off patent” in the next five years, which means competition will continue to intensify among brand name and generic drug makers for marketshare.  After patents on blockbuster drugs expire, the brand name manufacturers are trying new tactics. Merck’s Zocor cholesterol pill generated over $4 billion in sales in 2006.  After the Zocor patent expired, Merck announced plans in 2007 to cut prices of Zocor to try and stave off competition from incoming generics. Â
We’re likely to see more of these patent battles between the Pfizers and Ranbaxys of the world.
Â
Calgary – 11:00 MST
No commentsUS Patent Reform
For Canadian inventors and patent owners, the US is almost always the first jurisdiction in which patent protection is pursued.  Last Friday the U.S. House of Representatives passed a long-awaited comprehensive Patent Reform bill that will implement sweeping changes to the U.S. Patent system when it becomes law. The Senate is expected to pass their bill later this year. For a copy of the bill as passed thus far and further discussion, click here. Highlights include:
- Switch: from first-to-invent system to first-to-file system.
- Damages: damages would be tied to the “economic value [of the invention] properly attributable to the patent’s specific contribution over the prior art”, reducing many damage awards.
- Treble Damages:Â Â the instances where treble damages will be awarded are restricted, reducing damage awards.
- Post-Grant Review: post-grant review proceedings would be available within 12–months of issuance .
- Open Examination: Opening of the system for making submissions of prior art for any patent or pending application.
- Venue and Jurisdiction: Restrictions on venue would likely remove the popular E.D.Texas as a choice for patent infringement lawsuits.
Â
The progress of this bill is being closely watched and still hotly debated in the patent and licensing community in the US.
Calgary – 10:15 MST
Canadian Patent Update
The Canadian Intellectual Property Office has announced a few changes to patent practice this summer. These are a few of the highlights:
- Changes in Title: For changes in ownership that occurred prior to the filing of an application for a patent, applicants now need only provide a declaration (rather than evidence)Â of the chain of title.
- “Small Entity” Definiton: Small entities pay lower fees for filing. The definition of “small entity” for these purposes is now defined as an entity that employs 50 or fewer employees. Entities that are controlled directly or indirectly by an entity that employs more than 50 employees are excluded, as are entities that have entered into a license with an entity that has more than 50 employees. Universities also qualify as small entities by definition.
- Electromagnetic Signals: Claims to electromagnetic and acoustic signals are forms of energy and are not patentable. This does not apply to methods, processes, machines or manufactures involved in the generation, transmission, reception, or processing of such signals.
Â
Calgary – 11:10 MST
No commentsRolling out the Settlement Agreement: General Mills vs. Kraft Foods
 General Mills sells a rolled fruit snack under the trademark FRUIT BY THE FOOT, and has obtained not one but two patents: U.S. Patent Nos. 5,284,667 (“Rolled Food Item Fabricating Methods”) and 5,723,163 (“Rolled Food Item”). How mashed fruit and corn syrup can be a patentable invention is a separate issue.Â
General Mills sells a rolled fruit snack under the trademark FRUIT BY THE FOOT, and has obtained not one but two patents: U.S. Patent Nos. 5,284,667 (“Rolled Food Item Fabricating Methods”) and 5,723,163 (“Rolled Food Item”). How mashed fruit and corn syrup can be a patentable invention is a separate issue.Â
General Mills sued Farley, a rival maker of rolled fruit snacks, for patent infringement. The parties settled out of court, and the resulting agreement made it clear that Farley’s successors would benefit from the settlement terms if Farley sold its entire “rolled food product business”. Farley later sold its assets to Kraft Foods, and Kraft Foods in turn sold part of the Farley assets to a third company. General Mills then sued Kraft for patent infringement.
Did Kraft’s sale of part of the Farley assets strip Kraft of the protection in the settlement agreement, and expose it to a patent infringement suit by General Mills? The Federal Circuit Court of Appeals said no, in General Mills v. Kraft Foods (Fed. Cir. 2007) stating that there was nothing in the agreement to deprive Kraft of protection as Farley’s successor. General Mill’s “covenant not to sue” applied to Kraft as successor.
The lessons for business?
- When buying intellectual assets, ensure your lawyers review any past litigation and any Settlement Agreements to assess the scope of protection for successors;
- General Mills wanted to control the onward sale of assets through the settlement agreement – this is possible, but the terms must be clear in the settlement agreement and this case provides some guidelines on how that should be done;
- And yes, even rolled fruit can be an intellectual asset.
Â
Calgary – 09:54 MST
Â
No commentsUS Patent Decision: KSR v Teleflex
The US Supreme Court issued its decision on Monday in KSR International Co. v. Teleflex Inc. , a case which is considered by many commentators to be a watershed decision in US patent law.Â
KSR, a Canadian auto-parts company on contract to General Motors, designed a pedal with a sensor. Rival autoparts maker, Teleflex Inc., demanded royalties on the basis that the KSR pedal infringed a Teleflex patent for a pedal equipped with an electronic sensor. KSR refused to pay, asserting that the Teleflex patent was invalid since it had merely combined existing technologies in an obvious manner.
The court agreed with KSR, stating: “Granting patent protection to advances that would occur in the ordinary course without real innovation retards progress and may, in the case of patents combining previously known elements, deprive prior inventions of their value or utility.”
The practical impact of this decision is that many patents will now be open to challenge on obviousness grounds.  Inventions that introduce truly innovative concepts and elements will be more valuable than those which merely combine existing technologies in a new way.
Â
Calgary – 09:35 MST
Â
1 commentUS Patent License Decision
On Jan. 9, 2007, the US Supreme Court handed down its decision in MedImmune, Inc. v. Genentech, Inc. resulting in a significant change to the licensing landscape.  In this case, MedImmune had entered into a licensing agreement with Genentech under which MedImmune was to pay royalties for the use of Genentech’s “Cabilly I” patent and (when it issued) the “Cabilly II” patent.  When the second patent issued, Genentech sent a letter requiring the payment of royalties for the use of the second patent. MedImmune paid under protest, then turned around and sued the licensor on the basis that the second patent was not valid or enforceable. By continuing to make the payments, MedImmune kept the license alive.
The essential question in the case was whether a patent licensee was obliged to withhold its royalty payments and therefore commit a material breach of the license agreement before it had grounds to sue to declare the patent invalid? Prior case law said that a licensee did have to effectively breach the agreement before it had standing to sue. This decision overturns that line of cases and permits a licensee to challege the validity of the patent in court, while still making payments as a licensee and keeping the license. For MedImmune, breaching and terminating the license would have effectively shut down its own business.
For patent licensors, there are two practical points to remember:
1. Watch how you word your letters to licensees when politely requesting (or aggressively demanding) royalty payments, as such letters can be construed as a threat of litigation, providing grounds for a declaratory suit by the licensee;
2.  Consider reviewing language in the patent license to see if licensees can be forced to waive any right to challenge the validity of the licensed patent.
The fallout from this decision will be watched closely by both patent holders and their licensees.
Calgary – 12:32 MST
Â
No commentsPharma Patent Decision Released
Last week (November 3, 2006) the Supreme Court of Canada (SCC) issued a decison in the battle between AstraZeneca and Apotex on the issue of “evergreening” the protection available for pharmaceutical products. The decision cane be found at AstraZeneca Canada Inc. v. Canada (Minister of Health), 2006 SCC 49
The Patented Medicines (Notice Of Compliance) Regulations govern patented pharmaceutical products in Canada and provide brand-name drug companies with some extra tools in their competition against generic drug manufacturers. Here’s how it works in a nutshell:  a generic drug manufacturer cannot enter the market without a Notice of Compliance (NOC) and no NOC will be issued if the proposed product is the subject of a competitor’s patent. However, a generic can challenge that patent’s validity or applicability to its own product by issuing a notice of allegation. The brand name drug company (which owns the patent) may respond by issuing its own application for a prohibition of the NOC. This response results in an automatic 24‑month “statutory freeze” on the issuance of the NOC to the generic drug manufacturer. Even after a patent expires, by applying for new improvement patents of “marginal significance” to the expired patent, the brand-name drug company can obtain an indefinite series of 24‑month statutory freezes, which can effectively shut the generic out of the market for that product. There has been much criticism of this tactic.  This decision helps clarify the rules and warns against the use of the “evergreening” tactic by patent holders.Â
No commentsPersonal Liability of Director for IP Infringement
If a company is sued for infringement of intellectual property rights, can the director be held personally responsible? One of the benefits of incorporating a company is that the company’s shareholders and directors are not personally liable for the debts and liabilities of the company. That’s a basic proposition of corporate law. Of course, there are exceptions. In the 2006 decision in Krav Maga Enterprises, LLC v. Edge Combat Fitness Inc., the Federal Court allowed the addition of a director in a lawsuit for trade-mark infringement. Essentially, if the director or officer is engaged in “deliberate, willful and knowing pursuit of a course of conduct that is likely to constitute infringement or reflects an indifference to the risk of it” then the director can be tagged with personal liability. This is not a new idea. The Federal Court of Appeal has established this type of liability in cases such as Mentmore Manufacturing Co. Ltd. v. National Merchandise Manufacturing Co. (1978), 40 C.P.R. (2d) 164 in a case of patent infringement and another decision of the Federal Court adding a personal defendant in Dimplex North America Ltd. v. Globaltec Distributors Ltd. from 2005, a case also alleging patent infringement.Â
1 comment